Abstract
Background: Hematopoietic stem cells (HSC) ensure homeostasis and lifelong maintenance of hematopoietic system, but with age, they gradually lose quiescence, self-renewal potential, and system restoration capacity. HSC aging results in a differentiation shift towards myeloid lineage, anemia, thrombocytosis, decrease in T and B cells, imbalance in macrophage function, and increased osteoclast activity. Mechanisms involved in HSC aging include increased mTOR activity and ROS production, impaired autophagy, epigenetic reprograming, and cumulative DNA damage. Intriguingly, cellular and molecular similarities between aging and inflammation have led to a novel concept of "inflammation-associated aging of hematopoiesis". Understanding the molecular mechanisms responsible for this process may impact strategies targeting age-related diseases, including neoplasms. However, to date only few primary animal models of inflammation have shown bone marrow failure, so new animal models need to be established to provide mechanistic insight into the long-term implications of chronic inflammation on the hematopoietic system.
We have previously shown that bone marrow-specific deletion of an adapter protein Abelson interactor-1 (Abi1) leads to a myeloproliferative neoplasm (MPN)-like disease in 35-56-week-old mice, mechanistically associated with increased activity of Src Family Kinases (SFKs), STAT3 and NF-κB. At both transcript and protein levels, Abi-1 is also significantly reduced in HSCs and granulocytes from patients with primary myelofibrosis (PMF), and Abi-1-deficient HSC in human PMF show increased SFK-STAT3-NF-κB signaling (Chorzalska, ASH 2017).
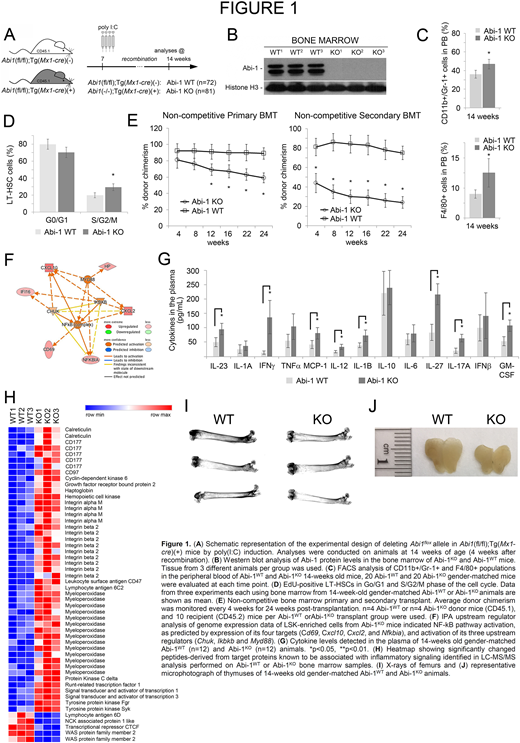
Methods: Myeloid/lymphoid, stem/progenitor populations profiling by FACS, bone marrow transplantation assays, transcriptomics and proteomics analyses as well pro-inflammatory cytokine profiling and histopathology analyses were performed on the transgenic Abi-1KO mice carrying bone marrow-selective knockout at 4 weeks post-recombination, upon confirming both inducible inactivation of the Abi1flox allele and loss of Abi-1 protein in the marrow (Fig.1A, B).
Results: To better understand initial systemic events that lead to the development of MPN-like disease in aged Abi-1KO mice we have now characterized early changes within the hematopoietic system associated with loss of Abi-1. Blood count analysis indicated leukocytosis, anemia and thrombocytosis, and an increase in the fraction of myeloid (CD11b+/Gr-1+) as well as macrophage/monocyte (F4/80+) cells at the expense of lymphoid (B220+) cells in Abi-1KO relative to Abi-1WT mice (Fig.1C). Previously reported 2.6-fold increase in Abi-1KO LT-HSCs (Chorzalska, ASH 2017) was now shown to be associated with 30% increase in number of LT-HSCs is in the S/G2/M phases of the cell cycle relative to Abi-1WT LT-HSCs (Fig. 1D). Lethally irradiated recipient C57BL/6 wild-type mice transplanted with bone marrow cells from Abi-1KO relative to Abi-1WT mice (in the absence of competitor cells) showed progressive loss of chimerism in primary and secondary recipients (Fig. E). Genome-wide gene expression analysis of Abi-1WT vs. Abi-1KO LSK cells showed significant overexpression of genes regulated by or involved in regulation of the NF-κB pathway (Fig. 1F). Plasma cytokine levels showed 2-fold increase in IL-1B, IL-12, IL-17, IL-23, IL-27, and MCP-1 and nearly 10-fold increase in INFγ (Fig. 1G). Label-free, intensity-based quantitative proteomic analysis of bone marrow from 20-week-old Abi-1KO and Abi-1WT mice showed abundance of peptides derived from Mac-1, myeloperoxidase, STAT1, STAT3, and SFKs - Hck and Fgr, confirming not only activation of SFKs and STAT3 signaling, but also increase in proteins associated with myeloid lineages (Fig. 1H). Loss of bone density (Fig. 1I) and a significant decrease in thymus size (Fig. 1J) were observed in Abi-1KO mice relative to Abi-1WT mice.
Conclusions: In sum, phenotypic analyses performed 4-10-weeks post Abi1 gene inactivation indicate changes consistent with accelerated aging of hematopoietic system that are mechanistically linked to inflammatory SFK-STAT3-NF-κB signaling. To our knowledge this is the first animal model linking accelerated inflammation-driven aging of hematopoietic system to development of an MPN in aged mice.
Olszewski:Genentech: Research Funding; TG Therapeutics: Research Funding; Spectrum Pharmaceuticals: Consultancy, Research Funding. Reagan:Pfizer: Research Funding; Alexion: Honoraria; Takeda Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal